The atomic mass is the mass of an atom. Atoms He Avogadros number Moles of He molar mass of He Mass of He.
Atomic Weight Of Helium Commission On Isotopic Abundances And Atomic Weights
Mass of 1 Helium-atom 0004N Kg.

What is the mass of helium atom. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Atomic mass of Lithium Li. Atomic mass of Hydrogen H.
Mass of one helium atomm4602310 23g. The mass of one atom of helium is equal to 400g. Atom Classification of Helium.
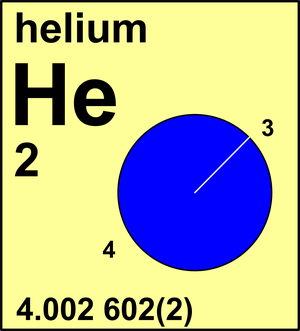
6886 views around the world. The atomic number of helium is 2. Helium with an atomic number of 2 and He as its element symbol is a unique element.
The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Combine equations 2-1 and 2-3 to get.
It has a relative atomic mass of 4 an atomic number of 2. By the definition the carbon atom with six neutrons denoted as carbon-12 has an atomic mass equal to 12 amu. Helium is a semi-metalmetalloid.
In 1969 the Commission recommended A r He 4002 601 which was identical to the atomic mass of 4 He to six significant figures. Atomic Number Element Atomic Mass u Atomic mass uRounded off 1. Atomic mass of Helium is 40026 u.
The flow of our calculation will be like this. Its average atomic mass is 4003 and has. Atomic mass of Helium is 40026 u.
What Is The Mass Of Exactly 1 Million Helium Atoms. Atomic Mass of Helium. The temperature of a perfect vacuum would be absolute zero because where there is.
Atomic mass of Helium is 40026 u. Where N Avagadro Number 6022 x 1023. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Therefore Mass of 1- Helium atom 00046022 x1023 66423 x 10-27 Kg. Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. Finding molar mass starts with units of grams per mole gmol.
Atomic mass of Beryllium Be. This isotope is present in natural sources of helium with a smaller abundance than that of any other stable isotope relative to its elemental composition. Atomic Mass of Helium.
Atomic Number of Helium. The atomic mass of Helium is 4002602. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
Find the average mass of a helium atom from its atomic mass A 4003 g mol 1 602210 23 atoms mol 1 1000 g kg 1 664710 27 kg. The atomic mass of helium He will give you the weight of one mole of this molecule. The atomic mass of Helium is 4002602.
Therefore Mass of 1- Helium atom 00046022 x1023 66423 x 10-27 Kg. Where N Avagadro Number 6022 x 1023. The molar mass of helium is 4g Determine the mass of a single atom of helium in kilograms 41000 x 602 x 1023 241 x 1021 kg This looks too Ask a question Log in.
Were being asked to determine the mass of one helium atom. Atomic Mass of First 20 Elements. Mass of helium atom in kilograms - WolframAlpha.
Volume of a cylinder. Atomic mass of Helium He. The atomic mass is the combined number of protons and neutrons in the atom.
Mass of 1 Helium-atom 0004N Kg. The atomic mass of a solitary atom is just its absolute mass and regularly expressed in atomic mass units or amu. Average velocity of a helium atom is 2410 2 ms240 ms.
The Atomic Mass Of Helium Is 400u. Helium belongs to group 8noble gases and period 1 in the periodic table. The chemical symbol for Helium is He.
The number of protons and the number of neutrons in helium are four. Molar mass of He from the periodic table 4003 gmol. 1 mol 400 g.
Difference Between Helium And Hydrogen Properties Isotopes Reactions Applications

Helium Element In Periodic Table Atomic Number Atomic Mass

Helium Atom Has An Atomic Mass Of 4u And Two Protons In Its Nucleus How Many Neutrons Youtube