The center of the triangle is 940 10-11 m from each hydrogen atom. The average distance between them is.
What Is The Ratio Of The Mass Of An Electron To That Of The Mass Of Hydrogen Atom Quora
The nitrogen-to-hydrogen atomic mass ratio is 139 and the nitrogen-to-hydrogen distance is 1014 10-11 m.

What is the mass of hydrogen atom in kg. Joules is the energy equivalent of this. Vote For Answer Like. Each mole has a Avogadros number of atoms or atoms.
9-26 the three hydrogen H atoms form an equilateral triangle. The Hydrogen Atom 12th April 2008 I. The mass of a hydrogen atom is.
7 1 0 2 7 k g distance between the two atoms 4 1 0 1 0 m. So the mass of One Hydrogen atom is 167 X 10 27 kg. With a standard atomic weight of 1008 hydrogen is the lightest element in the periodic table.
The reason for this effect is nuclear binding energy. Thus 1 kg hydrogen element or 1000 moles of hydrogen have atoms or 1000 times the Avogadros number. What is the mole of oxygen.
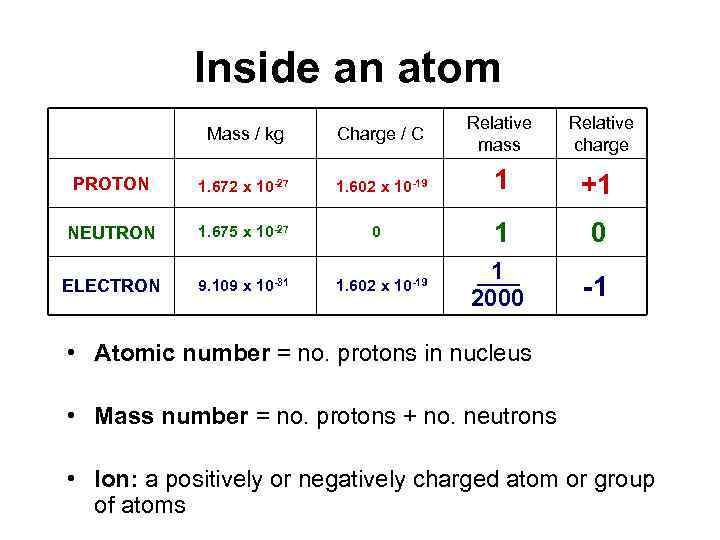
The hydrogen atom contains a proton mass 16710-27 kg and an electron mass 91110-31 kg. After all the protons in the nucleus are all positive and so the nucleus should just repel itself apart. Then how much does a hydrogen atom weigh in kg.
6023x1023 atoms of Hydrogen weigh 1008 g. If you think about it Hydrogen at 100794 is more than 112 of the weight of carbon-12 as you can see from the above table if you multiply 12 times the mass of a single hydrogen atom it comes to more than 12. V 43πr3 43π53 10-12m3 624 10-31m3 ρ mV 167 10-27kg624 10-31m3 27 103kgm3 27 kgdm3.
The mass of a hydrogen atom is 167 10-27kg. To make up 1 kilogram or 1000 g of hydrogen element 1000 moles of hydrogen is required. The hydrogen atom consists of a proton of mass 167x10-27 kg and an orbiting electron of mass 911x10-31 kg.
At 0C 32F or 27315K at standard atmospheric pressure. 1 cubic centimeter of Hydrogen weighs 0000082 gram g 1 cubic inch of Hydrogen weighs 0000047399 ounce oz Hydrogen weighs 0000082 gram per cubic centimeter or 0082 kilogram per cubic meter ie. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms.
Molar mass units Gram per mole gmol Kilogram per mole kgmol Standard atomic weight Hydrogen H Oxygen O Sulfur S Chlorine Cl Iron Fe Molecular mass Hydrogen molecule H2 Water molecule H2O Table salt sodium chloride. Nov 15 2021 So 1 atom of Hydrogen weighs 10086023x1023 g 0167x10-23 g 167 x10-27 kg. In one of its orbits the electron is 53x10-11 m from the proton.
Hydrogen as atomic H is the most abundant chemical element in the universe making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. Atomic mass Number of protons Number of neutrons. Atomic mass of Hydrogen is 10079 u.
C Speed of the light. The radius of a hydrogen atom calculated using self-consistent field functions is 53 pm or 53 10-12m. In kilograms the mass of a hydrogen atom is 167 times 10 to the power of negative 27 or 167 x 10-24 grams.
Of course this number is an approximation and due to the presence of isotopes and atomic forces involved in mass this number can be less than one percent more. Density of hydrogen is equal to 0082 kgm³. Atomic mass of hydrogen 1.
The atomic mass of hydrogen is 1 gmole. The Hydrogen Atom In this next section we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The nitrogen N atom is at the apex of a pyramid with the three hydrogen atoms forming the base.
What atom has most hydrogen. The dominant part of the interaction between the two particles is the electrostatic interaction. 7 1 0 2 7 k g Total mass of two molecules 2 1.
16605391 10 -25 kg. Mass and energy related together and that expression was given by the famous scientist named Albert Einstein. Here we can clearly see that mass of electron is 10000 times smaller than mass of proton so mass of electron is considered negligible.
A hydrogen atom is one mole of hydrogen atoms equal to one gram. Mass of hydrogen atom 1. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
E Energy related to mass m. M 0112 kg c. The atomic mass is the mass of an atom.
That conversion is based on one atomic measurement unit of atomic mass for an atom of hydrogen. 02214076 10 23. The hydrogen atom consists of a proton of mass m p 1710-27 kg and charge q e 1610-19 C and an electron of mass m e 9110-31 kg and charge -q e.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Type the number of Hydrogen molecule H2 you want to convert in the text box to see the results in the table. This will culminate in the de nition of the hydrogen-atom orbitals and.
To convert it into Kg multiply by 416610 27Kg. So 1 atom of Hydrogen weighs 10086023x1023 g 0167x10-23 g 167x10-27 kg. Most of the mass of the universe however is not in the form of chemical-element type matter but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.
How many moles is 16 grams of oxygen. Mass of hydrogen atoms in 1 kg of water 112 g 0112 kg. Atomic Mass of Hydrogen.
Hydrogen is the most abundant chemical substance in the universe constituting roughly 75. We now know that a hydrogen atom has a mass of 16735 x 10-24 grams and that the oxygen atom has a mass of 26561 X 10-23 grams. The mass of one proton is 167 X 10-27 kg and mass of one electron is 91 X 1031 kg.
Unit F 321 Module 1 2 1 Electron

The Mass Of A Hydrogen Molecule Is 3 32 10 27 Kg If 10 23 Hydrogen Molecules Strike Per Second A Fixed Wall Of Area 2 Cm 2 At An Angle Of 45 O To The Normal And Rebound Elastically With A Speed Of 10 3 M S Then The Pressure
