Thus 6023 x 10 24 atoms of Helium 1 6023 x 10 23 x 6023 x 10 24 moles 10 moles. Since number of particles 1 Avogadros number60210²³ atoms of He.

How Many Helium Atoms Are Contained In 3 7 Clutch Prep
602 1023 C atoms or 150 moles Al atoms.
What is the mass of 6 moles of helium atoms. Avogadros constant says that 1 mole of any atom contains 6022 1023 atoms. If we are given a mass or amount of a substance we can determine the number of moles in the substance. Mass of 1 atom 1 atom mass of a mole of atoms 6022 x 10 23 atoms.
One of the things this means is that a mole of Helium has a mass of 4003 grams. H 6626 10-34 J s the Boltzmann constant is 138 10-23 JK. The correct ans is 4 gram.
You can view more details on each measurement unit. Of atoms of Helium make 1 mole that is equivalent to molar mass of Helium approximately 4 grams. Atoms He Avogadros number Moles of He molar mass of He Mass of He.
Avogadros number gives us the number of entities present in 1 mole. One mole of oxygen 16grams and is 602x1023 atoms one milligram of oxygen is 0001 grams. 400 g mol 565 102mol 0226g.
We assume you are converting between grams Helium and mole. Well Avogadros number of helium atoms has a mass of 40g. Helium molecular weight.
The atomic mass of helium He will give you the weight of one mole of this molecule. Convert grams Helium to moles or moles Helium to grams Percent composition by element. Amazing discounts and special benefits seniors are entitled to.
340 1022 atoms 6022 1023 atoms mol 565 102mol. Moles 204 moles x 40 gmol 816 grams. 225 x 1025 atoms of lead 1 mole 2072g 774419g.
Determine the number of atoms in a 483 g sample of zinc. One mole of anything is 6022 X 1023 items. Notice that Helium gas consists of molecules of Helium that contain one atom of Helium.
The flow of our calculation will be like this. Molar mass of He 4002602 gmol. 1 mole 60 x 1023 atomsSo 3 mole 180 x 1023 atoms.
One mole of carbon is 6022 x 10 23 atoms of carbon Avogadros number. What is the de Broglie wavelength of the helium atoms that are moving at the root-mean-square speed. Refer to the atomic masses in the periodic table inside the front cover of this textbook.
The mass of 700 moles of a compound is determined to be 14007 g. And Avogadros number - 6022xx1023mol-1 Clearly we have half a mole of helium atoms so. What is the temperature in degrees Celsius of 523 moles of helium he gas confined to a volume of 523 L at a pressure of 523 atm-209 degrees Celsius.
The calculation needed to work out the mass of 1231024 of Helium atoms is a lot simpler if you leave out the 1024 until the end. 1 mol 400 g. 1 grams Helium is equal to 024983748071879 mole.
The atomic mass is the mass of onemole of the atom. A gas of helium atoms each of mass 665 10-27 kg are at room temperature of 200C. Mass of 1 atom mass of a mole of atoms 6022 x 10 23.
Number of molesnumber of particlesAvogadros number. We know by Avogadro number that 6023 x 10 23 no. The answer is 4002602.
6355 amu Hg 20059 amu S 3207 amu and He 400 amu. Number of moles mass used RMM of compound therefore number of moles 000116 625x10-5 moles. What is the mass of 225 x 1025 atoms of lead.
Mass of He atoms 817 g He. How many grams Helium in 1 mol. Molar mass of He from the periodic table 4003 gmol.
The atomic weight of Helium is 4003. The mass of a helium atom is 668X10-27 kg. From periodic table the molar mass of He is 40 gmol.
1 mole 602x1023 atoms of helium atoms in the gas phase has 3700 J of microscopic kinetic energy at room temperature. The atomic mass of helium He will give you the weight of one mole of this molecule. Molecular weight of Helium or mol The molecular formula for Helium is He.
6022xx1023helium atoms6022xx1023helium atomsmol-112mol And 12cancelmolxx400gcancelmol-1200g The units cancel appropriately to give me an answer in grams as required. 1 mol of an atom molecule or formula unit contains 602 x 10 23 atoms molecules or formula units 1 mol has a mass in grams equal to the atomic formula or molar mass of a substance. MOLE 1 mole PARTICLES ATOM MASS g 1 mole molar mass look it up on the PT 602 x 1023 atoms 1 mole 602 x 1023 atoms 1 mole atomic mass g Two Step Problems.
Were being asked to determine the mass of one helium atom. It is equal to the molar mass of the compound. According to the Harvard table of physical and astronomical constants the weight of 1 atom of helium is 400260325415 amu where 1 amu is 16605402-1024 grams.
Atoms of a Cu 6355 g c S 3207 g b Hg 20059 g d He 400 g. This is not the case for all elements. This refers to one mole of anything eggs paperclips atoms.
This relation is then used to convert a carbon atom to grams by the ratio. In this case you have 340 1022 atoms. Plug in the atomic mass of carbon to solve for the mass of 1 atom.
If we assume that all atoms move with the same speed what is that speed. 1 amu 1661 X 10-24g this is roughly equal to the mass of ONE proton Because the mass of one amu is so small chemists deal with a much larger number of atoms while working with chemicals. One mole is defined as 6022 X 1023.
1 mole Of Helium Weight of Helium4Weight of Helium 14 4 grams 1 mole of Helium contains 602310²³ Avogadro number atoms. Rosariomividaa3 and 22 more users found this answer helpful. The SI base unit for amount of substance is the mole.
Mass moles x molar mass of He. The mass of Avogadros number of atoms is the atomic mass expressed in grams. Calculate the mass of 1231024 helium atoms.
So its 3 moles. How many moles of atoms are in 120 gram of helium. 1 mol 400 gram.

What Is The Mass Of Helium Atom Whose Atomic Weight Is 4 003 G Mol Brainly Com
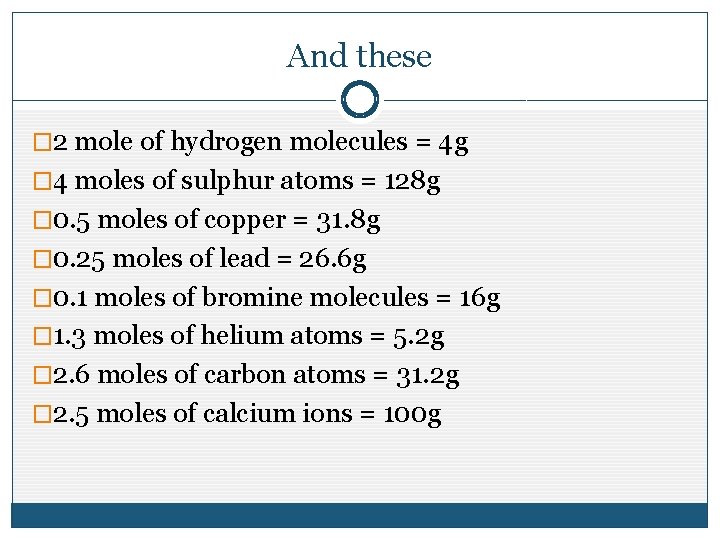
Moles Objectives To Understand What Is Meant By

How Many Moles Are 9 033 Xx 10 24 Atoms Of Helium He Youtube