Zinc is the reducing agent. Please calculate the reduced mass of hcl35hcl37dcl35dcl37.

Oxidation Reduction Redox Reactions Balancing Redox Reactions Chemistry Net Chemistry Classroom Chemistry Lessons Teaching Chemistry
H 66260755 10 34 joule sec c 299792458 10 8 m sec u 166054 10 27 kg pm 10 12 m mH 10078 u mCl 349688 u Calculate the reduced mass of HCl.

What is the reduced mass of hcl. 5 12. 4 HClg MnCl2aq 2 H2Ol. The reduced mass of two objects is a way to simplify a physics problem of two masses using one mass instead.
Cm dyne 5159x10. This compound is also known as Hydrochloric Acid. A What is the ratio of the reduced masses for the H2 and D2 The reduced mass is given by.
010 10 N HCl. 1 H 35 Cl 2 H 35 Cl. 5263 mL of 05 M H C l is shaken with 05 g of activated charcoal and filtered.
MH mCl mH mCl 1627 10 27 kg. Cm v cm r x cm. What is the equivalent mass of HCl in the given reaction.
Mass of HCl Mass of solution ww Mass of HCl 1180 365100. A balanced stomach s pH is generally 10-20. The reduced mass µ of a diatomic molecule A-B will be µ MaMb Ma Mb Where Ma is the molar mass of element A and Mb.
105 1017 ww solution. The concentration of the filtrate is reduced to 04 m. This low stomach fluid level normally leaves it free from microbes.
In the following reaction which element in what species is reduced. HCl molecular weight. However these terms dont depend on the reduced mass.
1 H 35 Cl. If deuterium is substituted for hydrogen however. Answer 1 of 3.
Hydrochloric acid concentration in the stomach is approximately 05 percent or 5000 parts per million. The following formula is used to calculate the reduced mass equivalent of two separate masses. M1 is the mass of object 1.
For H2 m1 m2 1 g mol-1 so μ H 5 5 g mol-1 ½ g mol-1. M2 is the mass of object 2. In this case HCl is reduced to H2- gas.
à - à. For D2 m1 m2 2 g mol-1 so μ H 6 6 g mol-1 1 g mol-1. B.
Relation if isotope effect and reduced mass check_circle Expert Answer. 2 H 35 Cl. E e e e.
Cm v cm r x cm. B O in NaOH. C H in HCl.
10502 304915 1280 10. 1 1. Amount of HCl 4307 g 365 g mol-1.
2 H 37 Cl. The result can then be used to calculate force and gravity effects as though there was a single mass at a point called the center of rotation. 2KMnO4 16 HCI 2KCI 2MnCl2 5Cl2 8H20 a 6.
As I understand it the series expansion of Vx where x is the displacement of the bond from equilibrium is Vx 1nd n Vdxx n. This is because the oxid. A solution of hydrogen chloride gas in water.
What is the reduced mass of hcl. The concentration of the filtrate is reduced to 04 M. Experts are tested by Chegg as specialists in their subject area.
Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. HCl gas is a mixture of H35Cl and H37Cl and the ratio of reduced masses for DCl is 10029 which might be observable.
When 182 g Zns is added a single displacement reaction is observed and the temperature rises to 305C. Here neither HCl or H- ion is the reducing agent. D Cl in HCl.
For the HCl molecule the needed reduced mass is Note that this is almost just the mass of the hydrogen. We review their content and use your feedback to keep the quality high. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
Mass of HCl 118 mol. Zn 2 HCl ZnCl2 H2. HCl and anharmonicity constant 0071 230198 323962.
And please let me know how the isotope of these atoms have an effect on reduced mass. μ à - à. The amount of adsorption of HCl per unit mass of adsorbent is.
202 001 N HCl. The chlorine is so massive that it moves very little while the hydrogen bounces back and forth like a ball on a rubber band. Reduced mass is calculated from the mass of both objects multiplied together then divided by the sum of the mass of the two objects.
M m₁ m₂ m₁ m₂ Where M is the reduced mass. 1 H 35 Cl 1 H 37 Cl. 1 H 37 Cl.
Weights of atoms and isotopes are from NIST article. Density 15 C4 C. NaOH aq HCl aq NaCl aq H₂Ol A Na in NaOH.
Molar mass of HCl 3646094 gmol. So anyway I understand why the ZPE of DCl is lower than that of HCl but Im not quite sure how anharmonicity causes k to not be constant. May be colored yellow by traces of iron chlorine and organic matter.
Fundamental constants conversion factors and atomic masses. 302 0001 N HCl. Because HCl amount is found in 1 dm 3 solution that HCl amount become the.
In a Chemical reaction a reducing agent reduces a substance and subsequently it will get oxidised. 5 2 and H. The equivalent mass of HCl in the given reaction is k2cr2o714hcl gives 2kcl2crcl33cl2h2o Solution f0--166 Equivalent weightHclnumber of atom oxidise or reducedf 365146 85167 85approx Suggest corrections.
Click hereto get an answer to your question 1. Mass of HCl 1180 365100 4307 g. X v x cm v cm.
110 01 N HCl. -1714 C 1081 solution. 100794 35453 Percent composition by element.
Reduced Mass 𝜇 kg Isotopes. Convert grams HCl to moles or moles HCl to grams. B.
Next amount of HCl is determined by dividing HCl amount from molar mass of HCl. A ff - p contains 1000 mL of 0300 M HCl at 203C. 9977 337252 1313 10.

Vibrational Spectra Of Simple Diatomic Molecules Vibrating Harmonically Spectroscopy Physical Youtube
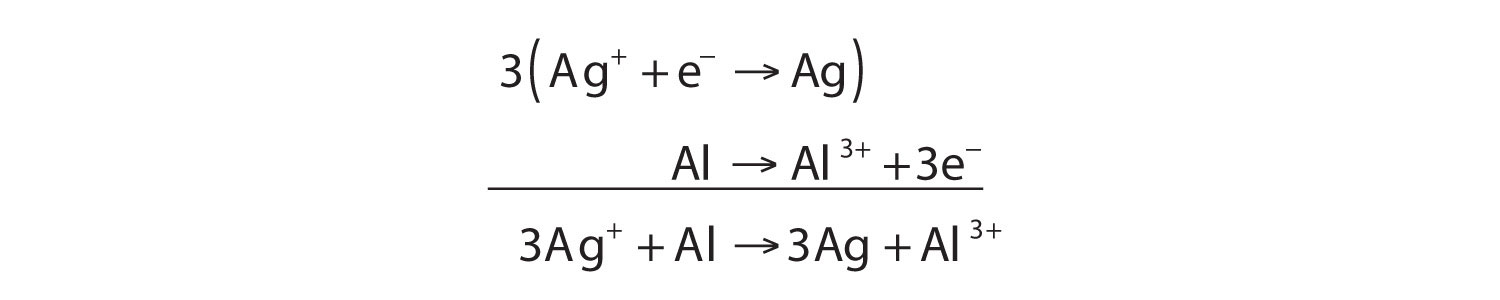
Oxidation Reduction Redox Reactions

Oxidation Agent Definition With Examples In Chemistry Chemical Reactions Chemistry Oxidation