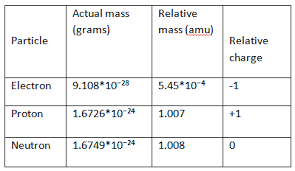
Both protons and neutrons have a mass of 1 amu and are found in the nucleus. They have negligible mass.
What Are The Exact Electron Location Found In Atom
Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or one Dalton.
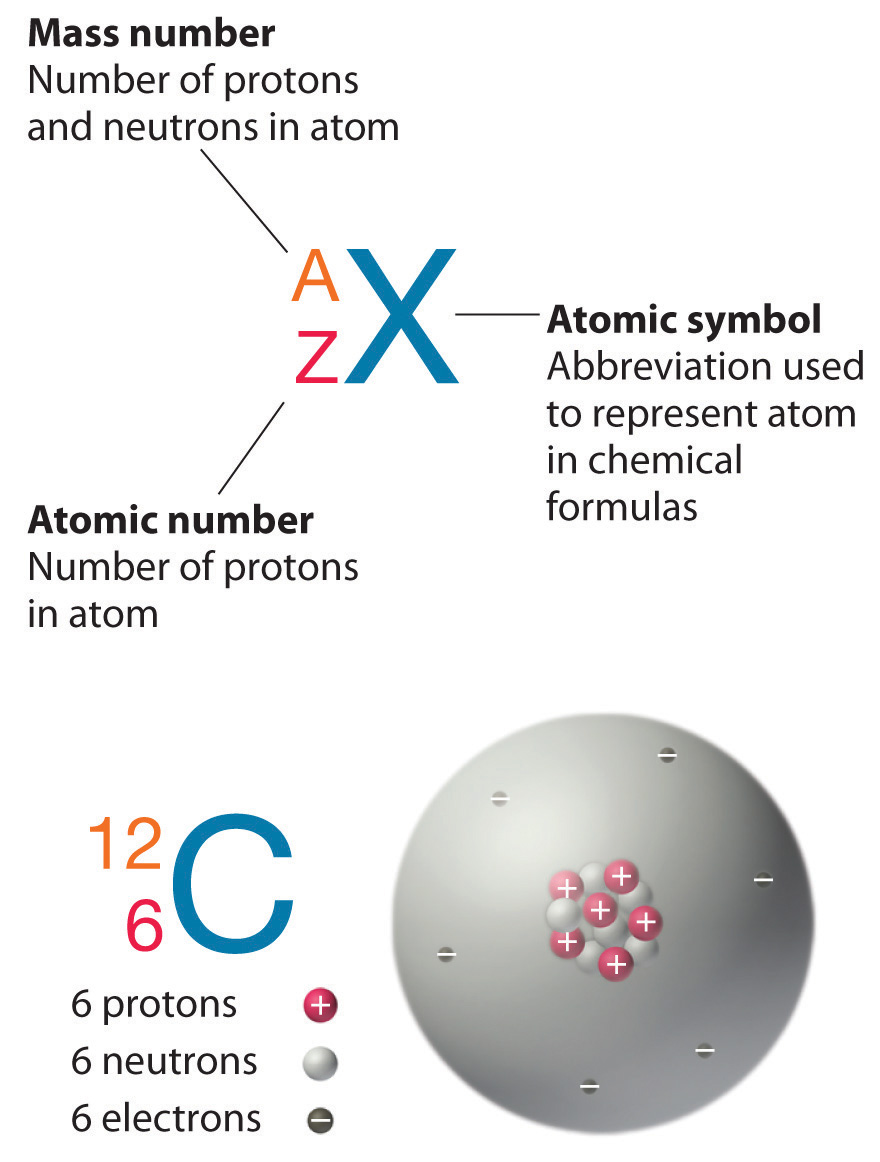
What is the exact mass of a neutron. Each electron has a negative charge -1 equal to the positive charge of a proton 1. The mass of Deuteron can be automatically converted to other mass units such as kilograms kg or mass of electrons via the pull-down menu. A They are exactly equal to each other.
B They are exactly equal to 1 amu. Mass of a neutron. However protons have a charge of 1 and neutrons are uncharged.
Thats why the neutrons in the diagram above are labeled n. The mass of neutron is equal to the mass of a proton. What is true about the mass of protons and neutrons.
The neutron was discovered in 1932 by the English physicist James Chadwick but since the time of Ernest Rutherford it had been known that the atomic mass number A of nuclei is a bit more than twice the atomic number Z for most atoms and that essentially all the mass of the atom is concentrated in the relatively tiny nucleus. One unified atomic mass unit is approximately the mass of one nucleon either a single proton or neutron and is numerically equivalent to 1 gmol. D They have masses slightly greater than 1 amu.
The mass of a neutron is slightly greater than the. U is equivalent to the dalton. Mass of neutron in AMU.
A numerical measure of a critical mass is dependent on the effective neutron multiplication factor k the average number of neutrons released per fission. Protons neutrons and electrons. Mass of neutron in grams.
Number of Neutrons Mass Number - Number of Protons 1 - 1 0. In kg mass of neutron 1674929 kg. Characteristics of a Neutron.
Its atomic mass is 56 and some change because the majority of the mass comes from the protons and neutrons which equal about 1 amu atomic mass unit while the electrons equal a fraction of a percent so just add up the protons and electrons to get the atomic mass henceforth the mass number. The mass of an electron is very very small compared to the mass of a proton and a neutron. The unified atomic mass unit symbol.
Neutron has no charge. 1 Fermi 1 fm 10-13 cm 10-15 m. When a nuclear chain reaction in a mass of fissile material is self-sustaining the mass is said to be in a critical state in which there is no increase or decrease in power temperature or neutron population.
The free neutron has a mass of 9395654133 eVc 2 or 167492747110 27 kg or 100866491588 u. Number of Neutrons 65 - 30 35. Electrons have a mass of approximately 0 amu orbit the nucleus and have a charge of -1.
They have masses slightly greater. The size of neutrons protons electrons is of the order 1 fermi. A neutron is represented by the symbol n.
They are exactly equal to each other. A hydrogen atom contains only one proton and one electron. One dalton is approximately the mass of one a single proton or neutron.
Mass of neutron 1008664 u or amu. Can anyone tell me if the radius. What is true about the mass of protons and neutrons.
Unlike protons and electrons which are electrically charged neutrons have no charge they are electrically neutral. Regarding this how is the mass of neutron determined. The unified atomic mass unit has a value of 1660 538 92173 1027 kg.
The neutron has a mean square radius of about 0810. For zinc the atomic weight is 6539 so the mass number is closest to 65. The amu without the unified prefix is an obsolete unit based on.
The Mass of a Deuteron Deuterium nucleus is 201355345 atomic masses u. Mass of neutron in MeV. The mass of the proton and the neutron are almost exactly the same.
Mass of neutron in kg. Or 16749 x 10-27 kg. They are exactly equal to 1 amu.
The absolute mass of a neutron is 16 10 27 kg. The relative mass of a neutron is 1 u. The above mentioned value is the mass of a proton 16749286X10-27 kg is the mass value of the heavier neutron.
Charge of an neutron. C They have negligible mass. Fermi is a unit of length.
The zero stands for zero charge. Neutrons are uncharged particles found within the nucleus. This constant defines the mass of deuteron the nucleus of Deuterium.
Using the exact masses of 115B 42He and 147N and the maximum energy of the neutron excited by the rays of polonium one may calculate for the neutron a mass 10068 taking 16O 161. For hydrogen 1008 is closer to 1 than 2 so lets call it 1. Mass of neutron 1840 x mass of electron.
Mass of neutron is 10086654 amu. When the unit is cm the unit 10-13 cm is read as fermi but when the unit used is metre the unit 10-15 m is read as femtometre. Neutron is 1842 times heavier than an electron.
For 12 C the atomic mass is exactly 12u since the atomic mass unit is defined from it.
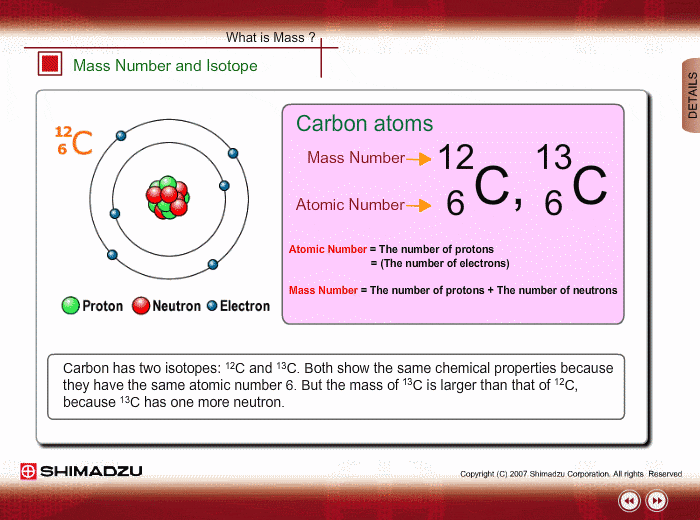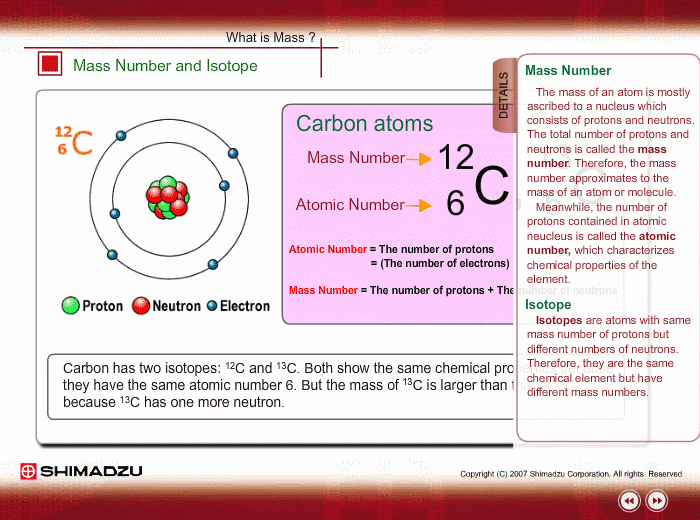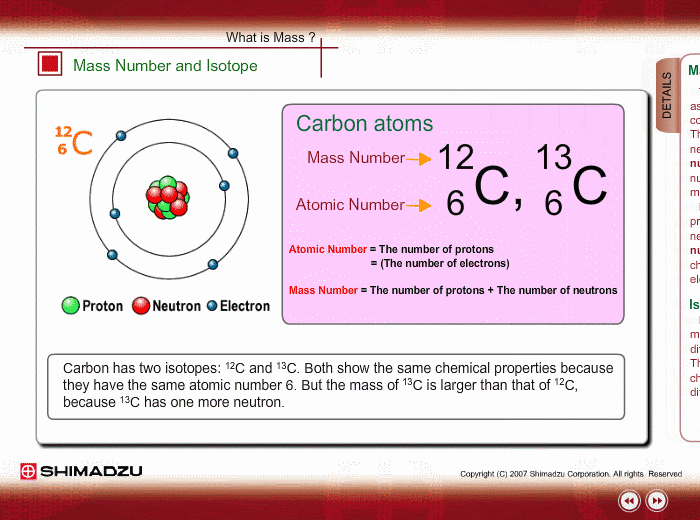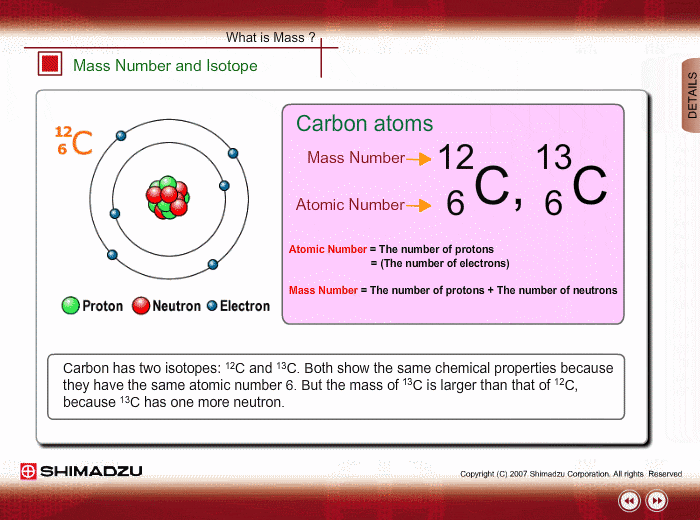
Mass Number And Isotope Shimadzu Shimadzu Corporation

Mass Spectrometry Ppt Download

If The Mass Of Neutron Is Doubled And Mass Of Electron Is Halved Then Find Out The New Atomic Mass Chemistry Structure Of Atom 12900827 Meritnation Com