Ten grams of LiOH will produce40 g LiBr LiOHaqHBraqrarrLiBraqH_2Ol Molar Masses of LiOH and LiBr LiOH1xx6941 gmol Li1xx15999 gmol O1xx100794 gmol H2394794 gmol LiOH LiBr1xx6941 gmol Li1xx79904gmol Br86845 gmol LiBr To complete this problem we are going to go from mass LiOH to moles LiOH to moles LiBr to mass LiBr. Using the limiting reactant 67 g of Fe in the reaction 04 mol of Fe304 was produced.

4 2 Formula Mass Percent Composition And The Mole Problems Chemistry Libretexts
2 C 4H10 g 13 O 2 g 8 CO 2 g 10 H 2O g Tro.

What is the mass of h20 produced when 10 grams. Calculate the speed of the third part. A stationary mass of 10 kg disintegrates explosively with the evolution of 42105 J into three parts two having equal masses of 3 kg. Quick conversion chart of grams H20 to mol.
2 moles H20 to grams 403176 grams. 5 moles H20 to grams 100794 grams. 1001802555 moles of H2O.
If 12 g of sodium metal is reacted with 2 mL of H20 how many grams of NaOH will be produced. What is the mass in grams of iron oxide produced. 540 - 320 220 If 72 grams of waterH20 and 64 grams of oxygen O2 are produced what mass of H2O2 decomposed.
XUnknown 90gGiven 2 KClO3s ----- 2 KCls 3 O2g 2 moles 3 moles X 90 g O2 1 mole O2 2 mole KClO3 1225 g KClO3 2297 g KClO3 32 g O2 3 moles O2 1 mole KClO3 I II III IV. 183 grams H2O 202 grams of H2 3604 grams of water 010257 grams of hydrogen. 10 g of H_2 is equivalent to 5 moles of hydrogen and 80 grams of oxygen is equivalent to 5 moles of oxygen.
4 mol H20 18 g H20 67 g Fe x 1 mol Fe x 1 mol Fe304 05 mol r-e304 04 mol r-e304 56 g Fe 3 mol Fe Therefore Fe is the limiting reactant. Answered 2 years ago. 3 moles H20 to grams 604764 grams.
For 2 moles of water we get 1 mole of O2. The combustion process for methane major component of natural gas is CH_g 209 à CO2g 2 H201 If 150 moles of methane are reacted what is the volume of carbon dioxide in L produced at 230C and. 9 moles H20 to grams 1814292 grams.
10 moles H20 to grams 201588 grams. 13 mol O 2. C 2 H 6 7 2 O 2--- 2CO 2 3H 2 O There is a molar ratio of 2 to 3 between carbon dioxide and water.
H2O is 1802 g per mole. 2 100 g O 2. 2 H 2 g O 2 g 2 H 2O l Limiting Reactants Chapter 4 Step 1.
H1 O16And the number of moles can be taken using the common equation nmMhere the m denoted the mass which given and the M is the molecular mass means how much mass in a one mole of molecule. 1 moles H20 to grams 201588 grams. 2C4H10 13O2 8CO2 10H2O.
40 grams H20 to mol 198425 mol. What mass in grams of KClO3 is consumed when 90 grams of O2 is produced according to the following reaction. 7 moles H20 to grams 1411116 grams.
N mM 80g32gmol 25mol. CaOH2aq H2g Cas 2 H200 HINT. So the answer should be.
Produced if there are 35 moles of oxygen. How many grams of water will be produced if there are 35 moles of oxygen. How many grams of SO 2 can be produced from reaction of 100 g of H 2 S and 100 g of O 2 as shown below.
A Molecular Approach 2e Mole ratio 2 mol C 4H10. Convert this to grams. A 621 C 308 E N one of the AnswerO 10 Which is the limiting reactant when 300 g af calcium are reacted with 800 g of water to produce hydrogen gas in the following equation.
Because the total mass of the reactantsstarting materials is equal to the mass of the products 20. If 100 grams of H 2 are mixed with 750 grams of O2 which reactant is the limiting reagent. So if we divide 100 g by 1802gmol we get how many moles of water molecules are present.
You need twice as the moles of H2 as O2 so there is insufficient H2 and it is the limiting reactant and all of it will react therefore 500 moles of H2 with. The molar mass of water is 18. H20 is the excess reactant.
1 Write the combustion reaction. 10 mol H 2O 2 molecules of C 4H10 react with 13 molecules of O 2 to form 8 molecules of CO 2 and 10 molecules of H 2O 2 moles of C 4H10 react with 13 moles of O 2 to form 8 moles of CO 2 and 10 moles of H 2O 2. How many grams of carbon dioxide are produced for each 800 grams of water produced.
Mol of O2 mol of H2O grams of H2O 35 mol O2 2 mol H2O 18 g H2O 130 g H 1 mol O 2O 2 1 mol H2O. A determine the mass of calcium hydroxide produced by each reactant. That means that for 0555 moles of water we get 0555 2 0278 moles of O2.
If 320 grams of nitrogen react completely with hydrogen and produce 540 grams of ammonia how many grams of hydrogen were used in the reaction. Convert mass to moles Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4. The number of moles of water produced is 5.
800 g of H20 5 mol. 100 grams H20 to mol 496061 mol. 2 H O 2 1008 1600 1802g.
6 moles H20 to grams 1209528 grams. 200 grams H20 to mol 992123 mol. 2 Determine moles in.
Next we know from the molecular formula that there are 2 parts of hydrogen H in a molecule therefore for every mole of water we have 2 moles of H. Mol of O2 mol of H2O 35 moles O2 2 moles H2O 70 mol H 1 moles O 2O 2 Mole to Mass. 2 H 2 S 3 O 2 fi 2 SO 2 2 H 2 O Assume H 2 S is LR.
4 moles H20 to grams 806352 grams. What is the molar mass gmol of 710 grams of gas whose volume is 540 L at 741 torr and 40C. Lets plug that into our equation.
1 grams H20 to mol 004961 mol. What mass in grams of hydrogen is produced by the reaction with 183g of wate knowing that the balanced equation predicts 1 mole of Hydrogen 202gmol will be produced from 3604 grams of water. First Find how much H2 gas is produced when 300 g of Ca is used.
20 grams H20 to mol 099212 mol. 10 grams H20 to mol 049606 mol. 100 g of O2 5 mol.
8 moles H20 to grams 1612704 grams. The molucular mass of H2O is 18gmol-. 2H₂ O₂ 2H₂O.
If you have 100 grams of butane and butanes molecular weight is M C4H1012411058 amu then the molarity will. 2 100 g H S 2 1 mol HS x 2 341 g HS 2 2 2 mol SO x 2 mol HS 2 0293 mol SO Assume O 2 is LR. 8 mol CO 2.
30 grams H20 to mol 148818 mol. 50 grams H20 to mol 248031 mol. These two masses move at right angles to each other.
Number of moles 100g 1802g 0555.

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy
If 100g Of Water Is Decomposed Then How Many Grams Of Oxygen And Hydrogen Are Obtained Quora
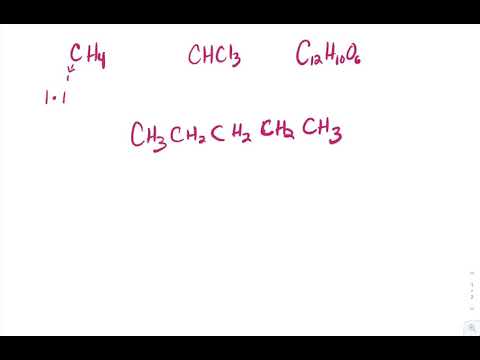
4 2 Formula Mass Percent Composition And The Mole Problems Chemistry Libretexts