4 52 1024 Avogadros number. Mass of 1 moleAvogadros number1gcancelmol-16022xx1023cancelmol-1 166xx10-24g.
What Is The Number Of Hydrogen Atoms In 3 G Of Hcl Quora
Wt of cl is 355gmol.

What is the mass of hydrogen atom in 1 mole hcl. We are to find the mass of the hydrogen atoms in 1 mole of water. In grams the mass of an atom of hydrogen is expressed as 167 x 10 -24. MOLAR MASS The mass of one mole of substance.
Find out the mass of Hydrogen atom and chlorine atom in 10 moles of HCI. The molar mass of water is 18 g mol-1 molar mass mass of one particle x Avogadros constant 602 x. Wt of H is 1gmol.
Mass of Cl 100 x mass of cl mass of HCl. From the mass number of hydrogen from the periodic table. Mar 13 2017.
Thus 1 kg hydrogen element or 1000 moles of hydrogen. So 1 mole of hydrogen weighs 2 grams. 4 moles HCl to grams 14584376 grams.
The Atomic Mass of. 1 molecule of HCl contains 1 hydrogen atom 12046X10 23 molecules of HCl contains12046X10 23 hydrogen atoms Calculation of number. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g.
So the number of moles of H atom in it. Get control of 2021. 5 moles HCl to grams 1823047 grams.
The atomic mass of hydrogen is 1 gmole. To make up 1 kilogram or 1000 g of hydrogen element 1000 moles of hydrogen is required. We can see from the above mentioned formula that water has 2 hydrpgen atoms.
Molar mass of hydrogen is 201588 000014 gmol. Track your food intake exercise sleep and meditation for free. Browse other questions tagged stoichiometry atoms mole or ask your own question.
1 Pressure of Hydrogen Gas. Each mole has a Avogadros number of atoms or atoms. 8 moles HCl to grams 29168752 grams.
3 moles HCl to grams 10938282 grams. In kilograms the molar mass of a hydrogen atom can be written as 0001 kilograms per mole. It has units of g mol-1 or kg mol-1.
2 moles HCl to grams 7292188 grams. 7 moles HCl to grams 25522658 grams. Atomic Mass of Atoms.
We know that the formula of water is. Using your pressure temperature and volume data you will calculate the mass of hydrogen gas formed in the reaction. So 1 mole of CH 4 will have 4 mol atom of Hydrogen.
An amu is equivalent to one dalton unit. Convert between H2 weight and moles. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H.
Hence one mole of Hydrogen gas eqH_2 eq will have 2 grams of mass whereas one. This is done in three steps. Once the number of moles of a substance present is known we can use.
100 x 355365. The mass of an atom of hydrogen can also be expressed in molar mass units as one gram per mole. 1 pressure of H 2 using Daltons Law 2 moles using the ideal gas law and 3 grams using molar mass as outlined on page 4.
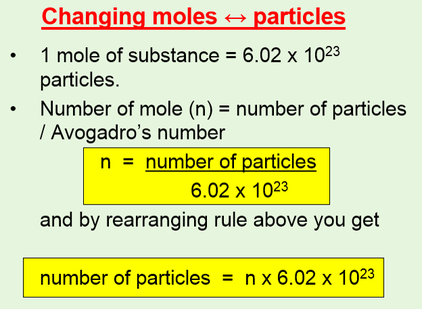
Molar mass to find the number of grams Avogadros number to find the number of atoms ions or molecules Moles B Grams B Atoms B Molar mass NA. The molar mass of Hydrogen Chloride HCl is 3646 gmol It is made up of equal parts of Hydrogen molar mass 1007 and Chlorine molar mass 35453. MOLES The mole the standard unit of amount of a substance mol the number of particles in a mole is known as Avogadros constant NA Avogadros constant has a value of 602 x 1023 mol-1.
An analysis indicated that the concentration was 068 parts per billion. Thus weight of 1 atom of hydrogen is 60210 231008. Hydrogen contained in 10 moles of HC 1 1 10 10 mol Clatom 10 1 10 mol.
One mole of hydrogen atoms has a mass of 1008 g so one mole of hydrogen gas would have a mass of twice this mass 2016 g. 2 6023 1023 this is the approximate mass of the H molecule. Compound name is hydrogen.
Moles of O 700 g of C 6H 12O x 1mol C H O6 x 6 mol O Molar mass Molar ratio 1801 g mol C6H12 O6 233 mol O Strategy. From the periodic table we get to know that Hydrogen has an atomic mass of 100794 grams. Molar mass of hydrogen.
Now just divide. 6 moles HCl to grams 21876564 grams. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
And thus to find the mass of 1 atom of hydrogen we take the quotient. Find out the number of moles of hydrogen and Oxygen atoms present in 10 moles of HCI. One mole HC 1 contains 1 mole of hydrogen and 1 mole of chlorine.
The same as the mass of one mole of the compound in grams. The level of mercury in a stream was suspected to be above the minimum considered safe 1 part per billion by weight. 60210 23atoms of hydrogen weigh 1008 g.
As there are 2 atoms of hydrogen in water so grams is the answer. Well to a first approximation one mole of hydrogen atoms has a mass of 1gAgreed. Assume a density of 10 gmL and calculate the molarity of mercury in the stream.
1 moles HCl to grams 3646094 grams. Atomic weight Atoms Mass percent. Mass 0f Cl9726.
52 1024 Avogadros number. Now number of moles of CH 4 in 52 1024 molecules is. Hydrogen has an atomic mass of 1 amu and a molar mass of 1 gram per mole.
Featured on Meta Please welcome Valued Associates 999 - Bella Blue 1001 - Salmon of Wisdom. In this regard why is the atomic mass of hydrogen 1.

Which Of The Following Have Larger Number Of Atom 1 1 Mole Of Nh3 2 2 Mole Of Hcl Chemistry Topperlearning Com Q4i1ja44