Take 12 Tesla-FT-MS result out of MS mz876330 suspect MNa adduct read M853340782 enter this value into formula finder with 2 ppm mass accuracy CHNSOP enabled get some thousand results compare isotopic pattern get happy. 1-1 p a peak in the mass spectrum.
I n m M.
How to calculate mass from m/z. Using the molecular ion to find the relative formula mass. A few compounds have mass spectra which dont contain a molecular ion peak because all the molecular ions break into fragments. Mz 142 142 142 142 unit mz exact mass 1421723 1420264 1420743 1419911.
You can find the mass M by the formula mz M nxprotonsn where n is the charge. Add or remove hydrogen atoms and adjust the charge accordingly to simulate gain or loss of protons and to use a positive value or negative value for the charge. Use your calculator and list of major mz peaks to determine numerical m values.
For example if ceC7H72 arose by the loss of two electrons the mz would be 4552683903 the ion mass would be 9105367806 and the molecule mass would be 9105477522. Formula C 2 H 5 O Mass 45 amu Charge 1 mz 45. Approximate mz 18 m141111z1 The value of z does NOT have to always be 1.
Mass moles molar mass. Write these values down in your notebook. Exact Masses and Molecular Formulae How to determine a molecular formula from an exact mass.
1 Combining the two previous equations yields. It is best understood as calculated from the dimensionless mass number m of a given ion and the number of its elementary charges z. When presenting data in a mass spectrum it is common to use the dimensionless mz which denotes the dimensionless quantity formed by dividing the mass number of the ion by its charge number.
Monolinuron MH Monolinuron dimer M Centroid and continuum spectra overlaid. M mass of the pure substance measured in grams g n amount of the pure substance measured in moles mol This mathematical equation can be rearranged to give the following. Look up in a table or From R.
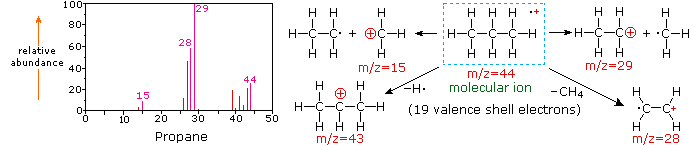
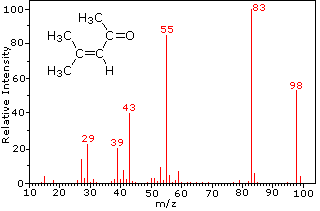
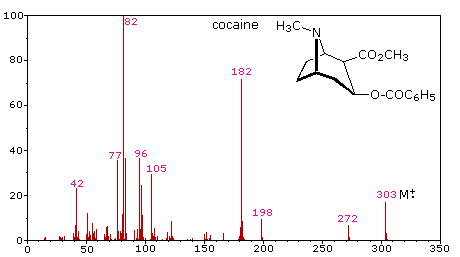
The mass-to-charge ratio mz read m over z is dimensionless by definition. Calculate the mass mz value of the molecular ion M for each of these structures. N m M.
Isotope spacing is 05 mz and isotopic resolution is not achieved. 6 pts Br но CH3 0 NH2 d H3C e Br N-CI O CH3 0 2. Taxol C47H51NO14 M85333089 Enter 85333089 in green box read M229 mz876320108 2 Reverse.
What does mz practically mean. Molecular Mass Calculator. Step 1 Understand that the peak value observed in the spectrum equals mass divided by charge and that the mass is the mass of the molecule plus any adducts protons 1901 masscharge and 3792 masscharge.
Masses in mass spectrometry are given as mass divided by charge of the molecule mz. DBE and Z - calculate the double bond equivalents DBE or hydrogen deficiency Z from a molecular formula. The theoretical mz of the monoisotopic peak for a given molecular formula.
So to get the neutral molecule mass you will have to add back the missing electrons or substract the gained electrons from the calculated ion mass to get the molecule mass. You must always remember that you are not measuring the mass of a neutral molecule in MS it is the mz value. Mz MnHn MH Singly charged ion.
Use the simplified mass spectrum from Step 2 to determine the mass difference m between each peak and the next peak on the spectrum. Mz 30 NO2 O NO CO mz 93 mz 65 NO2 NO2 C4H3 HH mz 77 mz 51. Calibration HelpeR - create reference lists for calibrating mass spectra.
Nominal mass mass calculated using the integer mass of the most abundant isotope for each element H1 C12 O16 N14 etc 2. Whenever symmetry can be used to determine a center-of-mass coordinate we avoid doing a triple integral. 5 Molecular Ion parent ion M molecular mass of the analyte.
To calculate the moles of pure substance. 1 1 d d 1 d d d m v M M x y z x y z M ρ ρ rcm r r r i j k r cm cm cm 1 d d d 1 d d d 1 d d d x x x y z M y z x y z M z z x y z M ρ ρ ρ r r r. When calculating the theoretical mz remember to include the charge carriers eg.
Monolinuron MH mass -89. Mass Spectroscopy-Determination of Structure Measurement of Mass M to Charge Ratio Z MZ r mvqBr radius m mass v velocity q charge B Magnetic Field Klein Organic Chemistry 3e. Formula C 3 H 7 Mass 43 amu Charge 1 mz 43.
These two equations can be rewritten. Webster Spectrometric Identification of Organic CompoundsWiley 1998 6th. Mass Spectroscopy-Determination of Structure Measurement of Mass M to Charge Ratio Z MZ.
Choosing from the structures a- f in question 1 above state all of the structures which. Moles mass molar mass ii m n M. In the mass spectrum the heaviest ion the one with the greatest mz value is likely to be the molecular ion.
M total mass of an ion. The mass spectrometer gives the mass to charge ratio mz therefore the sample. Note the 1 mz isotope spacingDoubly charged M2H 2 ion.
The mz range for an mz of interest plusminus a specified error in ppm. Mass 88 amu Charge 1 mz 88. This web application calculates the molecular mass average monoisotopic and nominal the elemental composition and the mass distribution spectrum of a molecule given by its chemical formula relative element weights or sequence.
Always use the mass of the most abundant isotope of each element for this sort of calculation.
12 3 Interpreting Mass Spectra Chemistry Libretexts
12 3 Interpreting Mass Spectra Chemistry Libretexts